Conformational isomerism
So, you know how we have different shapes of toys, like we have cubes and balls and cars, right? And because they have different shapes, we can play with them in different ways.
Well, just like that, molecules also have different shapes. And when a molecule can have different shapes, we call it "flexible".
Now, some molecules can actually change their shape on their own, without us doing anything to them. We call this "conformational isomerism". It's like a toy that can change shape by itself!
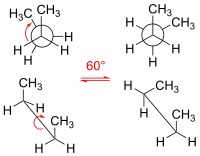
Here's an example: let's take a molecule called butane. It's made up of 4 carbon atoms and 10 hydrogen atoms. Now, if we draw it out, it looks like a long snake with a carbon atom at every turn.
So, if we were to hold this snake at one end and twist it, we'd change the shape of the snake, right? Well, molecules can do something similar. But because they're so tiny, we can't see it with our eyes. Instead, scientists use models to show how the different conformations look.
In the case of butane, it can twist around its central carbon-carbon bond. When it does this, it can either be in a "staggered" conformation or an "eclipsed" conformation.
Think of it like this - imagine you have two snakes (one is butane in the staggered conformation, and the other is butane in the eclipsed conformation). You place them side by side, with their tails touching. If you look at them from the top, the staggered snake will look like it's peeking out from between the coils of the eclipsed snake.
So, just like how different toys have different shapes we can play with, molecules like butane can have different shapes they can "play" in (or conformations).
Well, just like that, molecules also have different shapes. And when a molecule can have different shapes, we call it "flexible".
Now, some molecules can actually change their shape on their own, without us doing anything to them. We call this "conformational isomerism". It's like a toy that can change shape by itself!
Here's an example: let's take a molecule called butane. It's made up of 4 carbon atoms and 10 hydrogen atoms. Now, if we draw it out, it looks like a long snake with a carbon atom at every turn.
So, if we were to hold this snake at one end and twist it, we'd change the shape of the snake, right? Well, molecules can do something similar. But because they're so tiny, we can't see it with our eyes. Instead, scientists use models to show how the different conformations look.
In the case of butane, it can twist around its central carbon-carbon bond. When it does this, it can either be in a "staggered" conformation or an "eclipsed" conformation.
Think of it like this - imagine you have two snakes (one is butane in the staggered conformation, and the other is butane in the eclipsed conformation). You place them side by side, with their tails touching. If you look at them from the top, the staggered snake will look like it's peeking out from between the coils of the eclipsed snake.
So, just like how different toys have different shapes we can play with, molecules like butane can have different shapes they can "play" in (or conformations).
Related topics others have asked about:
