Isomer
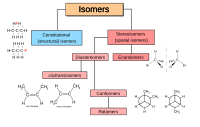
Alright kiddo, let's talk about isomers!
Imagine you have some Lego blocks. You can put them together in many different ways to create different structures, right? Well, molecules in chemistry are kind of like Lego blocks too. They are made up of atoms that can be arranged in different ways to create different chemicals.
Now, an isomer is a type of molecule that has the same atoms as another molecule, but they are arranged differently. It's like making two different shapes with your Lego blocks by rearranging them.
For example, let's say we have two molecules called butane and isobutane. Both of these molecules have the same four atoms (two carbons and six hydrogens), but the way they are arranged is different. Butane has the carbons in a straight line, while isobutane has one carbon branching off to the side.
Even though these molecules have the same atoms, they can have different properties. For example, isobutane is used as a fuel while butane is used in lighters.
So there you have it, a simple explanation of isomers! They are just molecules with the same atoms arranged in different ways, and can have different properties because of it.
Imagine you have some Lego blocks. You can put them together in many different ways to create different structures, right? Well, molecules in chemistry are kind of like Lego blocks too. They are made up of atoms that can be arranged in different ways to create different chemicals.
Now, an isomer is a type of molecule that has the same atoms as another molecule, but they are arranged differently. It's like making two different shapes with your Lego blocks by rearranging them.
For example, let's say we have two molecules called butane and isobutane. Both of these molecules have the same four atoms (two carbons and six hydrogens), but the way they are arranged is different. Butane has the carbons in a straight line, while isobutane has one carbon branching off to the side.
Even though these molecules have the same atoms, they can have different properties. For example, isobutane is used as a fuel while butane is used in lighters.
So there you have it, a simple explanation of isomers! They are just molecules with the same atoms arranged in different ways, and can have different properties because of it.
Related topics others have asked about:
