Boltzmann's entropy formula
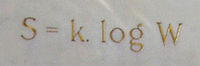
Have you ever played with a bunch of marbles or Legos and tried to count how many different ways you can arrange them? Well, Boltzmann's entropy formula is like a super cool way for scientists to figure out how many different ways you can arrange tiny particles called atoms and molecules in a big space.
First, let's start with the idea of entropy. Entropy is a fancy word that basically means disorder or randomness. Imagine you have a big box of Legos and you dump them all out on the floor. At first, they might be neatly stacked on top of each other, but as you start to move them around, they become more and more disordered. That is entropy.
Now imagine that instead of Legos, you have tiny particles like atoms or molecules in a box. Boltzmann's entropy formula helps us figure out how many different ways we can arrange those tiny particles so that they have a certain amount of energy. The formula looks like this:
S = k ln W
Don't worry, we'll break it down. S stands for entropy, k is Boltzmann's constant (which is just a number scientists use), and ln W means the natural logarithm of W.
So what is W? W represents the number of different ways we can arrange our tiny particles so that they have a certain amount of energy. For example, if we have 10 particles in our box and we want to arrange them so that they have a total energy of 100, there might be a few different ways we can do that. Maybe we have five particles with an energy of 20 and five particles with an energy of 10, or maybe we have two particles with an energy of 50 and eight particles with an energy of 5. All of those different arrangements are part of W.
The natural logarithm of W is just a fancy way of saying "take the log of W using a certain mathematical constant." This helps scientists make the formula work better, but you don't really need to worry about all that.
So what does the formula actually tell us? Basically, it helps us figure out how likely certain arrangements of particles are based on their energy. If there are a lot of different ways to arrange the particles with a certain amount of energy, then the entropy is higher, because there are more possible ways things can be disordered. If there are only a few ways to arrange the particles, then the entropy is lower.
Overall, Boltzmann's entropy formula is a really important tool for scientists trying to understand how tiny particles behave in different environments. But don't worry if you don't quite get it yet – it's a pretty complicated idea for a five-year-old!
First, let's start with the idea of entropy. Entropy is a fancy word that basically means disorder or randomness. Imagine you have a big box of Legos and you dump them all out on the floor. At first, they might be neatly stacked on top of each other, but as you start to move them around, they become more and more disordered. That is entropy.
Now imagine that instead of Legos, you have tiny particles like atoms or molecules in a box. Boltzmann's entropy formula helps us figure out how many different ways we can arrange those tiny particles so that they have a certain amount of energy. The formula looks like this:
S = k ln W
Don't worry, we'll break it down. S stands for entropy, k is Boltzmann's constant (which is just a number scientists use), and ln W means the natural logarithm of W.
So what is W? W represents the number of different ways we can arrange our tiny particles so that they have a certain amount of energy. For example, if we have 10 particles in our box and we want to arrange them so that they have a total energy of 100, there might be a few different ways we can do that. Maybe we have five particles with an energy of 20 and five particles with an energy of 10, or maybe we have two particles with an energy of 50 and eight particles with an energy of 5. All of those different arrangements are part of W.
The natural logarithm of W is just a fancy way of saying "take the log of W using a certain mathematical constant." This helps scientists make the formula work better, but you don't really need to worry about all that.
So what does the formula actually tell us? Basically, it helps us figure out how likely certain arrangements of particles are based on their energy. If there are a lot of different ways to arrange the particles with a certain amount of energy, then the entropy is higher, because there are more possible ways things can be disordered. If there are only a few ways to arrange the particles, then the entropy is lower.
Overall, Boltzmann's entropy formula is a really important tool for scientists trying to understand how tiny particles behave in different environments. But don't worry if you don't quite get it yet – it's a pretty complicated idea for a five-year-old!
Related topics others have asked about:
