Covalent radius
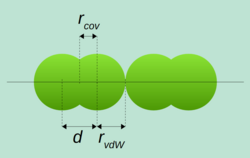
Covalent radius is the size of an atom in a molecule. When atoms link together to form molecules, the atoms need to get very close to each other. The covalent radius is a measure of how big the atoms get when they link together. It's like when you link two pieces of dough together — they need to get close enough so they can stick together, but they don't have to get too close or they'll be mushed together too much. The covalent radius tells us how close the atoms have to get to link up and form a molecule.
Related topics others have asked about:
