Enthalpy of vaporization
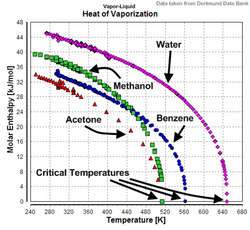
Enthalpy of vaporization (or "heat of vaporization") is a measure of how much energy it takes to turn a liquid into a gas or vapor. It's like measuring how much energy it takes to make something "boil". For example, when a pot of water is heated, the heat energy goes into turning the water into steam. The amount of energy that it takes to turn liquid water into steam is called its enthalpy of vaporization.
Related topics others have asked about:
