Electrochemical cell
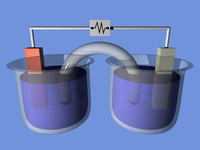
An electrochemical cell is like a magic box that turns chemicals into electricity. Imagine you have two cups, one with juice and another with water. Now, you take a straw and connect these two cups by putting it in both cups. If you blow air into one cup, the fluid will move to the other cup. This happens because of the difference in pressure between the two cups. An electrochemical cell works in the same way but instead of using air, it uses chemicals.
Let us talk about the structure of an electrochemical cell. It has two electrodes, one called the anode and the other called the cathode. The anode is usually made of a reactive metal, and the cathode is usually made of a less reactive metal. These electrodes are dipped into an electrolyte solution, which is a liquid that allows the flow of charged particles called ions.
When you connect the two electrodes with a wire, a current starts flowing. Here's how it works: The reactive metal on the anode starts losing electrons, which are negatively charged particles. These electrons travel through the wire and reach the cathode. The less reactive metal on the cathode attracts these electrons and starts gaining them. This exchange of electrons creates a flow of electric current.
At the same time, the ions in the electrolyte solution also participate in this reaction. The positively charged ions move towards the negatively charged electrode, and the negatively charged ions move toward the positively charged electrode. This movement of ions creates a reaction that balances out the charge, enabling the reaction to continue.
So, in summary, an electrochemical cell is a device that uses two electrodes dipped in an electrolyte solution to convert chemical energy into electrical energy. The movement of electrons from the anode to the cathode and the corresponding movement of ions in the solution creates a flow of electric current.
Let us talk about the structure of an electrochemical cell. It has two electrodes, one called the anode and the other called the cathode. The anode is usually made of a reactive metal, and the cathode is usually made of a less reactive metal. These electrodes are dipped into an electrolyte solution, which is a liquid that allows the flow of charged particles called ions.
When you connect the two electrodes with a wire, a current starts flowing. Here's how it works: The reactive metal on the anode starts losing electrons, which are negatively charged particles. These electrons travel through the wire and reach the cathode. The less reactive metal on the cathode attracts these electrons and starts gaining them. This exchange of electrons creates a flow of electric current.
At the same time, the ions in the electrolyte solution also participate in this reaction. The positively charged ions move towards the negatively charged electrode, and the negatively charged ions move toward the positively charged electrode. This movement of ions creates a reaction that balances out the charge, enabling the reaction to continue.
So, in summary, an electrochemical cell is a device that uses two electrodes dipped in an electrolyte solution to convert chemical energy into electrical energy. The movement of electrons from the anode to the cathode and the corresponding movement of ions in the solution creates a flow of electric current.
Related topics others have asked about:
