Electrodiffusion
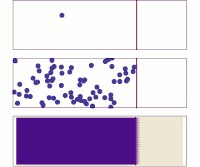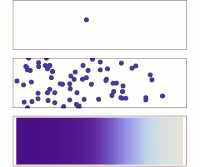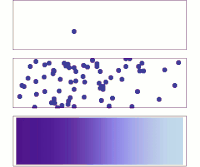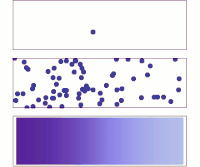
Hey kiddo, electrodiffusion is when electrically charged particles move around by themselves and mix together. Let's imagine that you have a cup of water and some salt. You put the salt in the water and stir it until it dissolves.
Now, the water has salt ions floating around in it - positively charged sodium ions and negatively charged chloride ions. But these ions don't just sit there - they move around randomly. Sometimes a sodium ion will bump into a chloride ion and stick to it, forming a neutral salt molecule. Then, maybe a few seconds later, another salt molecule will break apart into a sodium ion and a chloride ion again.
This random movement of charged particles is called diffusion. It happens because particles naturally want to spread out and mix together until the concentration is even all over. But what if you added an electric field to this situation?
An electric field is a force that pushes charged particles in a certain direction. So if you put a positive charge on one side of the cup and a negative charge on the other, the sodium and chloride ions will start to move towards their respective charges. This is called electrophoresis.
But something really interesting happens when you combine electrophoresis with diffusion - you get electrodiffusion! The charged particles are now moving around randomly AND being pushed by the electric field. This means that they will move much faster in one direction than if they were just diffusing on their own.
Electrodiffusion is really important in things like biological cells, where charged ions need to move through channels in the cell membrane. It's also used in chemistry and materials science to control the movement and behavior of particles in solutions. Pretty cool, huh?
Now, the water has salt ions floating around in it - positively charged sodium ions and negatively charged chloride ions. But these ions don't just sit there - they move around randomly. Sometimes a sodium ion will bump into a chloride ion and stick to it, forming a neutral salt molecule. Then, maybe a few seconds later, another salt molecule will break apart into a sodium ion and a chloride ion again.
This random movement of charged particles is called diffusion. It happens because particles naturally want to spread out and mix together until the concentration is even all over. But what if you added an electric field to this situation?
An electric field is a force that pushes charged particles in a certain direction. So if you put a positive charge on one side of the cup and a negative charge on the other, the sodium and chloride ions will start to move towards their respective charges. This is called electrophoresis.
But something really interesting happens when you combine electrophoresis with diffusion - you get electrodiffusion! The charged particles are now moving around randomly AND being pushed by the electric field. This means that they will move much faster in one direction than if they were just diffusing on their own.
Electrodiffusion is really important in things like biological cells, where charged ions need to move through channels in the cell membrane. It's also used in chemistry and materials science to control the movement and behavior of particles in solutions. Pretty cool, huh?
Related topics others have asked about:
