Electrolytic cell
Imagine you have a toy car that needs a new battery to run. You go to the store and buy a new battery, but it doesn't work until you insert it into the car the right way - with the positive side facing the right direction. This is because batteries work by creating an electrical charge, which provides energy to power the car.
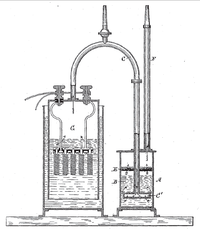
An electrolytic cell is like a special kind of battery. Instead of using a chemical reaction to create a charge, an electrolytic cell uses electricity to create a chemical reaction. The process is a bit like magic: by passing an electric current through a liquid or a gas, we can cause different chemicals to separate or combine, which can create new substances or produce energy.
To make an electrolytic cell, we need three things: an electrolyte (a liquid or gas that can conduct electricity), two electrodes (conducting rods made of metal or graphite), and an external power source (like a battery or a power supply). When we connect the electrodes to the power source and dip them into the electrolyte, the magic begins.
One electrode (called the anode) gets a positive charge, while the other electrode (called the cathode) gets a negative charge. This creates a flow of electrons through the electrolyte, which can cause different chemical reactions to occur. Depending on the type of electrolyte and electrodes we use, we can create many different effects.
For example, if we use an electrolytic cell to split water (H2O) into hydrogen (H2) and oxygen (O2), we need to use electrodes made of a conductive metal (like platinum) and a source of electricity with enough voltage to break the water molecules apart. When we dip the electrodes into a container of water with a bit of salt or acid added (to make it conductive), bubbles of gas start to appear on each electrode. The gas on the anode is hydrogen, while the gas on the cathode is oxygen - just like the two components of water!
Electrolytic cells are used in many different applications, from producing pure metals and chemicals to purifying water and creating energy storage systems. Understanding how they work can help us design new materials and technologies that help us solve problems and improve our lives.
An electrolytic cell is like a special kind of battery. Instead of using a chemical reaction to create a charge, an electrolytic cell uses electricity to create a chemical reaction. The process is a bit like magic: by passing an electric current through a liquid or a gas, we can cause different chemicals to separate or combine, which can create new substances or produce energy.
To make an electrolytic cell, we need three things: an electrolyte (a liquid or gas that can conduct electricity), two electrodes (conducting rods made of metal or graphite), and an external power source (like a battery or a power supply). When we connect the electrodes to the power source and dip them into the electrolyte, the magic begins.
One electrode (called the anode) gets a positive charge, while the other electrode (called the cathode) gets a negative charge. This creates a flow of electrons through the electrolyte, which can cause different chemical reactions to occur. Depending on the type of electrolyte and electrodes we use, we can create many different effects.
For example, if we use an electrolytic cell to split water (H2O) into hydrogen (H2) and oxygen (O2), we need to use electrodes made of a conductive metal (like platinum) and a source of electricity with enough voltage to break the water molecules apart. When we dip the electrodes into a container of water with a bit of salt or acid added (to make it conductive), bubbles of gas start to appear on each electrode. The gas on the anode is hydrogen, while the gas on the cathode is oxygen - just like the two components of water!
Electrolytic cells are used in many different applications, from producing pure metals and chemicals to purifying water and creating energy storage systems. Understanding how they work can help us design new materials and technologies that help us solve problems and improve our lives.
Related topics others have asked about:
