VSEPR theory
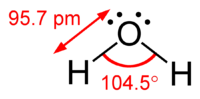
VSEPR theory is a way to understand how atoms in a molecule or ion arrange themselves in space. Imagine playing with blocks, like Legos. Each block is like an atom, and they can attach to each other in different ways to make different shapes.
The VSEPR theory says that atoms in a molecule or ion try to spread out as much as possible in order to minimize repulsion between their electron clouds. Think of the electron cloud as a fluffy cushion that surrounds each atom. When two atoms get too close together, their electron clouds start to bump into each other and push each other away.
So, when atoms come together to form a molecule or ion, they arrange themselves in a way that allows their electron clouds to spread out as much as possible. There are different shapes that molecules and ions can take, and these shapes depend on the number of atoms and the types of atoms involved.
For example, if there are just two atoms, they will arrange themselves so that they are as far apart as possible. This gives them a linear shape, like a straight line. If there are three atoms, they will arrange themselves in a triangular shape, like a pyramid.
The VSEPR theory is helpful because it allows us to predict the shape of a molecule or ion before we even create it. This helps us to understand how the molecule or ion will behave, and how it will interact with other molecules and ions.
The VSEPR theory says that atoms in a molecule or ion try to spread out as much as possible in order to minimize repulsion between their electron clouds. Think of the electron cloud as a fluffy cushion that surrounds each atom. When two atoms get too close together, their electron clouds start to bump into each other and push each other away.
So, when atoms come together to form a molecule or ion, they arrange themselves in a way that allows their electron clouds to spread out as much as possible. There are different shapes that molecules and ions can take, and these shapes depend on the number of atoms and the types of atoms involved.
For example, if there are just two atoms, they will arrange themselves so that they are as far apart as possible. This gives them a linear shape, like a straight line. If there are three atoms, they will arrange themselves in a triangular shape, like a pyramid.
The VSEPR theory is helpful because it allows us to predict the shape of a molecule or ion before we even create it. This helps us to understand how the molecule or ion will behave, and how it will interact with other molecules and ions.
Related topics others have asked about:
