Combined gas law
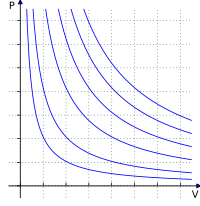
The combined gas law is like a recipe for how gases behave when they are heated up or cooled down or when they experience pressure changes.
Imagine you are trying to bake a cake and you need to adjust the recipe to make more or less cake. Similarly, the combined gas law helps us adjust the recipe for gases when we change the conditions they are in.
The three important factors that affect how much space gases take up are temperature, pressure, and volume. When you change one of these, it can affect the others. So, the combined gas law tells us how everything works together.
Think of it like playing with Play-Doh. If you squeeze a big ball of Play-Doh, it gets smaller, and it gets harder to push your fingers into it. That's like changing the pressure of a gas. If you add heat to the Play-Doh, it gets softer and easier to manipulate. That's like changing the temperature of a gas.
Now, let's say you have a certain amount of Play-Doh, and you want to see how much space it takes up if you squeeze it really hard and heat it up at the same time. The combined gas law is like a tool that helps you figure it out.
So, in short, the combined gas law is a way to measure how gases behave when they experience different amounts of pressure, temperature, and volume.
Imagine you are trying to bake a cake and you need to adjust the recipe to make more or less cake. Similarly, the combined gas law helps us adjust the recipe for gases when we change the conditions they are in.
The three important factors that affect how much space gases take up are temperature, pressure, and volume. When you change one of these, it can affect the others. So, the combined gas law tells us how everything works together.
Think of it like playing with Play-Doh. If you squeeze a big ball of Play-Doh, it gets smaller, and it gets harder to push your fingers into it. That's like changing the pressure of a gas. If you add heat to the Play-Doh, it gets softer and easier to manipulate. That's like changing the temperature of a gas.
Now, let's say you have a certain amount of Play-Doh, and you want to see how much space it takes up if you squeeze it really hard and heat it up at the same time. The combined gas law is like a tool that helps you figure it out.
So, in short, the combined gas law is a way to measure how gases behave when they experience different amounts of pressure, temperature, and volume.
Related topics others have asked about:
