D-block contraction
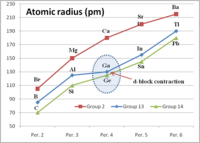
Okay kiddo, so the d-block contraction is like when you build a tower with blocks. Imagine the blocks are the elements in the middle of the periodic table, like nickel and copper.
When you build the tower, you always start with the biggest blocks at the bottom and the smallest blocks at the top. So with elements, the biggest ones are at the bottom of the d-block, like zinc and cadmium.
But as you move up the tower, the blocks get smaller and smaller, right? That's kind of what happens with the elements in the d-block as you move from the bottom to the top of the periodic table.
The d-block contraction says that even though the elements are getting smaller as you move up the table, the differences in size get smaller and smaller as well. So the elements get closer and closer in size to each other as you move up.
This can affect how the elements behave in different reactions and chemical processes, just like how the way you build the tower can affect how it stands up and if it falls over.
When you build the tower, you always start with the biggest blocks at the bottom and the smallest blocks at the top. So with elements, the biggest ones are at the bottom of the d-block, like zinc and cadmium.
But as you move up the tower, the blocks get smaller and smaller, right? That's kind of what happens with the elements in the d-block as you move from the bottom to the top of the periodic table.
The d-block contraction says that even though the elements are getting smaller as you move up the table, the differences in size get smaller and smaller as well. So the elements get closer and closer in size to each other as you move up.
This can affect how the elements behave in different reactions and chemical processes, just like how the way you build the tower can affect how it stands up and if it falls over.
Related topics others have asked about:
