Electrochemical gradient
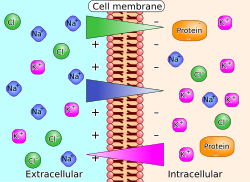
Imagine a bowl of jellybeans. Each jellybean is like a tiny piece of electricity that wants to move around. Now, imagine that you split the bowl in half and put a tiny hole in the middle. Some of the jellybeans will want to move from one side of the bowl to the other through that hole because they have different flavors or colors.
The electrochemical gradient is kind of like that bowl of jellybeans, but instead of jellybeans, it's made up of atoms and molecules that have different charges, or electrical properties. These charges create a force that makes the particles want to move around.
In our body, cells use an electrochemical gradient to get things in and out of the cell. For example, our cells need to take in things like sugar and nutrients, and get rid of waste. The electrochemical gradient helps with this process by creating a force that pulls the things we need into the cell and pushes the waste out.
Overall, the electrochemical gradient is a fancy way of describing how particles with different electrical properties create a force that causes them to move around. This force helps our cells do important things like taking in nutrients and getting rid of waste.
The electrochemical gradient is kind of like that bowl of jellybeans, but instead of jellybeans, it's made up of atoms and molecules that have different charges, or electrical properties. These charges create a force that makes the particles want to move around.
In our body, cells use an electrochemical gradient to get things in and out of the cell. For example, our cells need to take in things like sugar and nutrients, and get rid of waste. The electrochemical gradient helps with this process by creating a force that pulls the things we need into the cell and pushes the waste out.
Overall, the electrochemical gradient is a fancy way of describing how particles with different electrical properties create a force that causes them to move around. This force helps our cells do important things like taking in nutrients and getting rid of waste.
Related topics others have asked about:
