Enantiomers
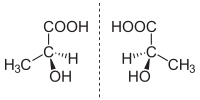
Enantiomers are molecules that are almost exactly the same as each other, but are actually different in a very important way. Think of it like having a pair of gloves - they look the same, but one is meant for the left hand and the other for the right hand. Just like gloves, enantiomers have the same basic shape, but they are "handedness" and can't be superimposed on top of each other.
In science, we call these "handedness" versions of a molecule mirror images of each other. Like when you look in a mirror, your reflection looks like you, but flipped. This is the same for enantiomers.
So, what makes them different? Well, for some molecules, their mirror images react differently with other molecules in the body. So, imagine you have two ice cream cones - one goes to the right hand and the other goes to the left hand. Even though they both look the same, they'll go to different places, right? Enantiomers can do the same thing.
This affects how the body processes them. One enantiomer might be helpful and "fit" with the molecules in our body, while the other may not do that and be useless or harmful. This is why enantiomers are important to make sure that the medicine we take is the correct version and will work properly in our body.
In science, we call these "handedness" versions of a molecule mirror images of each other. Like when you look in a mirror, your reflection looks like you, but flipped. This is the same for enantiomers.
So, what makes them different? Well, for some molecules, their mirror images react differently with other molecules in the body. So, imagine you have two ice cream cones - one goes to the right hand and the other goes to the left hand. Even though they both look the same, they'll go to different places, right? Enantiomers can do the same thing.
This affects how the body processes them. One enantiomer might be helpful and "fit" with the molecules in our body, while the other may not do that and be useless or harmful. This is why enantiomers are important to make sure that the medicine we take is the correct version and will work properly in our body.
Related topics others have asked about:
