Levorotation and dextrorotation
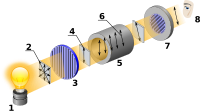
Imagine holding a long stick in your hands. If you turn the stick clockwise, you are rotating it to the right, and if you turn the stick counterclockwise, you are rotating it to the left. This is called rotation.
Now, imagine that instead of a stick, you have a molecule. Molecules can also rotate, but instead of clockwise or counterclockwise, they can rotate to the left or to the right. This is called levorotation and dextrorotation.
Levorotation means that the molecule is rotating to the left, just like if you were rotating the stick counterclockwise. Dextrorotation means that the molecule is rotating to the right, just like if you were rotating the stick clockwise.
Scientists use these terms to describe molecules that have a certain type of structure. Molecules that rotate to the left are called "levorotatory" or just "levorotatory", and molecules that rotate to the right are called "dextrorotatory" or just "dextrorotatory".
So, imagine you have a bunch of molecules in a solution, and some of them are levorotatory, and some of them are dextrorotatory. Scientists can figure out which ones are which by measuring how much they rotate the light that passes through the solution. The amount of rotation is different for levorotatory and dextrorotatory molecules, just like the stick would rotate differently if you turned it to the left or to the right.
In summary, levorotation and dextrorotation are terms used to describe the rotation of molecules to the left or to the right, just like if you were rotating a stick counterclockwise or clockwise. Scientists use these terms to help identify the structure of different molecules.
Now, imagine that instead of a stick, you have a molecule. Molecules can also rotate, but instead of clockwise or counterclockwise, they can rotate to the left or to the right. This is called levorotation and dextrorotation.
Levorotation means that the molecule is rotating to the left, just like if you were rotating the stick counterclockwise. Dextrorotation means that the molecule is rotating to the right, just like if you were rotating the stick clockwise.
Scientists use these terms to describe molecules that have a certain type of structure. Molecules that rotate to the left are called "levorotatory" or just "levorotatory", and molecules that rotate to the right are called "dextrorotatory" or just "dextrorotatory".
So, imagine you have a bunch of molecules in a solution, and some of them are levorotatory, and some of them are dextrorotatory. Scientists can figure out which ones are which by measuring how much they rotate the light that passes through the solution. The amount of rotation is different for levorotatory and dextrorotatory molecules, just like the stick would rotate differently if you turned it to the left or to the right.
In summary, levorotation and dextrorotation are terms used to describe the rotation of molecules to the left or to the right, just like if you were rotating a stick counterclockwise or clockwise. Scientists use these terms to help identify the structure of different molecules.
Related topics others have asked about:
